![The Chemistry Inside Those Tiny Champagne Bubbles [Infographic]](https://www.distillerytrail.com/wp-content/uploads/2017/12/the-chemistry-inside-those-tiny-champagne-bubbles-infographic-cover.jpg)
More bottles of Champagne are popped around New Years than any other time of year. It’s hard to believe but, Champagne and Bourbon have some similarities. Just like all bourbon is whiskey but not all whiskies are bourbon, all Champagne is sparkling wine but not all sparkling wines are Champagne. In order to legally be labeled bourbon, the whiskey among other things, must be made in the United States and contain at least 51% corn. In a similar fashion, to legally be labeled Champagne, the sparkling wine must be made from grapes grown in the geographic region of Champagne, France.
 Régions de France
Régions de France

Diving Into Champagne’s Bubbles
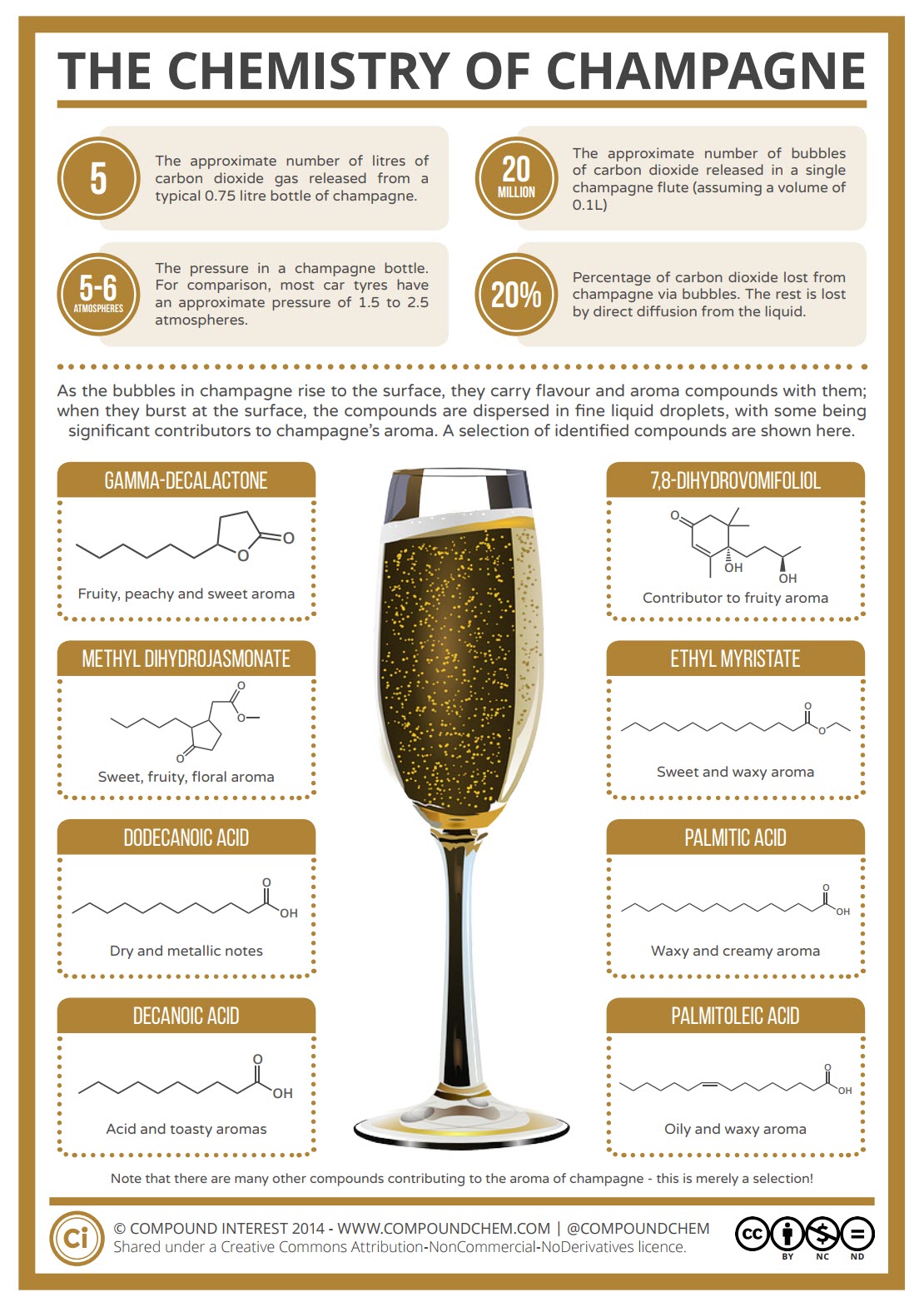
We are not going to go into all the chemical details of Champagne but, we will touch on the chemistry that happens as the bubbles are released once the bottle opens. The obvious chemical contributor that causes the bubbles to appear in champagne in the first place is carbon dioxide, which originates from the fermentation process. Champagne is unusual amongst wines, in that it undergoes two fermentations – one before bottling, and one in the bottle before it is drunk. The second fermentation produces the carbon dioxide and ethanol that are vital for the finished product.

As the bubbles reach the surface and burst, these compounds can be thrown into the air within tiny liquid droplets. Scientists have analyzed the composition of these droplets, collected by holding a microscope slide over a champagne glass then transferring them to a solution which was then run through a spectrometer to identify compounds present. A large number of flavor and aroma compounds were discovered in the droplets, a selection of which are shown in the Infographic including,
- Gamma-Decalactone – Fruity, peachy and sweet aroma
- Methyl Dihydrojasmonate – Sweet, fruity, floral aroma
- Dodecanoic Acid – Dry and metallic notes
- Decanoic Acid – Acid and toasty aromas
- 7,8-Dihydrovomifoliol – Contributor to fruity aroma
- Ethyl Myristate – Sweet and waxy aroma
- Palmitic Acid – Waxy and creamy aroma
- Palmitoleic Acid – Oily and waxy aroma
Hundreds of components were present, with some still yet to be identified, but interestingly, the composition of these droplets differs from that of the main body of the wine. These are merely a selection of the many compounds found in champagne.
The Chemistry Inside Champagne Bubbles Infographic
Click anywhere on the infographic to see it full size.
The Chemistry of Champagne Video
What’s the best way to pour a glass of Champagne?
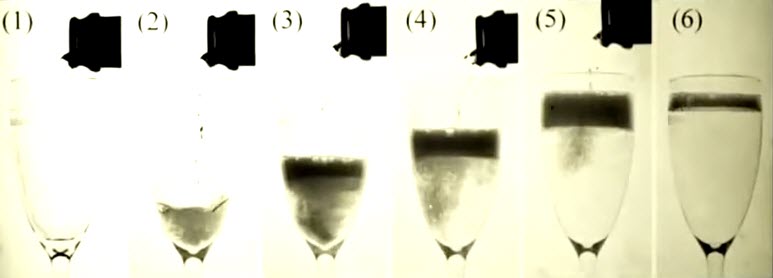
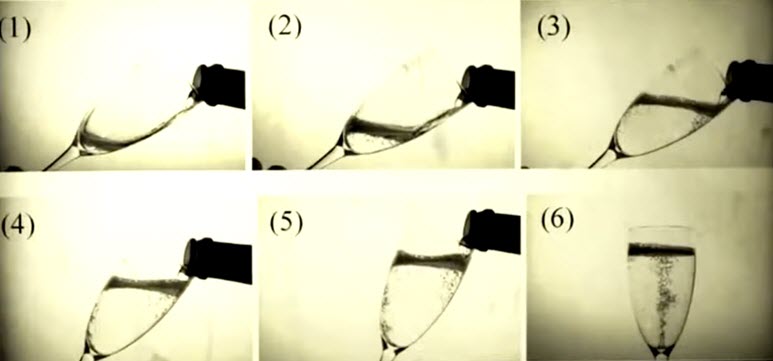
In order to maximize the sensory experience from a glass of Champagne you must pour the Champagne properly. According to a study published in American Chemical Society Journal of Agricultural and Food Chemistry pouring Champagne at an angle preserves up to twice the carbon dioxide bubbles vs. pouring Champagne directly down the glass. This helps to maximize the flavor and unique sensory experience of Champagne. (Click to enlarge.)
Enough about science get out there and ring in the New Year with a nice glass of sparkling wine from the Champagne region of France. Then, starting January 2, it’s back to distilled spirits!
If your into this topic and would like to learn more about food trivia and science, you can check out this book, Why Does Asparagus Make Your Pee Smell?: Fascinating Food Trivia Explained with Science.Please help to support Distillery Trail. Sign up for our Newsletter, like us on Facebook and follow us on Twitter.
Resource: The Chemistry of Champagne