Vodka is tasteless, odorless and colorless. Just makes you want to go out and get you some right? But, it’s also the No. 1 distilled spirit in the world. Not necessarily because of what it is but because it’s so easy to mix with other things to make for a tasty cocktail. And then there are those that prefer it neat or on the rocks.
Whether You Spell it Potato or Potatoe it’s All Potatoes Right? Wrong!
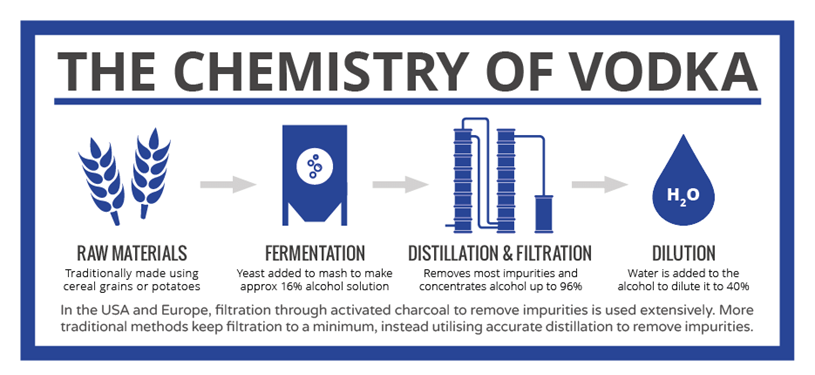
There’s a common misconception that Vodka is made from potatoes. Some Vodka is however; it’s less than 1%. Most modern Vodkas are made from cereal grains including corn, rye and wheat. But, it doesn’t stop there, it could also be made from sourgum, rice, molasses, soybeans, grapes, rice, sugar beets or other grains. Today’s craft distilleries love to experiment with Vodka because the grains are so flexible and it does not require time to mature like a Bourbon. That’s important when you are in startup mode and you need to get some cash flow going!
Fermentation of these grains using yeast produces alcohol (ethanol), but only up to around 16% – too low for vodka. Further steps are necessary to arrive at the finished product, the key step being distillation. Distillation involves the boiling of the mixture; because ethanol boils at 78˚C, it boils off before the water does and it can therefore be concentrated. Unfortunately, a lot of the other compounds produced during fermentation boil off at lower temperatures than water too, so precise control of the distillation process is necessary to ensure that these aren’t present in the final product.
Stay Informed: Sign up here for the Distillery Trail free email newsletter and be the first to get all the latest news, trends, job listings and events in your inbox.
Don’t They All Taste the Same Then?
No, due to differences in distillation and the way ethanol and water interact in different vodkas. As well as existing as individual molecules, the water and ethanol molecules can form structures called hydrates. These are cage-like structures, with a number of the water molecules surrounding an ethanol molecule.
The most common of these hydrate structures in vodka has around 5 water molecules to each ethanol molecule. Researchers discovered that its concentration varied in different brands of vodka. Though their hypothesis has yet to be conclusively confirmed, they speculate that these structural differences in different vodkas could account for slight differences in taster perceptions.
Take a look at this Infographic and you’ll see that the ethanol hydrates, impurities left in or removed by filtration and additives can all affect the final product that is produced.
Click the Chemistry of Vodka image to view the full size image.
Please help to support Distillery Trail. Like us on Facebook and Follow us on Twitter.





